Steps for Implementing a Quality Management System—the Successful Way!
In quality management, it’s a popular saying that if something is not written, it didn't happen. Businesses which follow a management system need a well-conceived and systematized Quality Management System (QMS). Many organizations failed to manage a QMS while others managed it well.
What is a QMS and why is it Important?
QMS consists of written and controlled guidelines and procedures that form a foundation for all procedures. An organized QMS ascertains the steps for key processes and forms methods in preventing failures in a timely manner. QMS is organized to protect the brand, organization processes, and the customers' interest.
What are the benefits of implementing a QMS?
Implementation of quality management system should result in many long-term financial gains. Here is the list of few benefits of effective implementation of a QMS:
- Achieve organizational goals.
- Reduce costly errors.
- Improve customer satisfaction.
- Market your business more effectively.
- Manage growth more effectively.
- Improve documentation availability.
- Correct issues to improve products and services.
- Grow market share in new territories and market sectors.
- Creates a culture of quality.
- Embed vision for all projects.
- Better internal communications.
- Consistent products.
- Measure performance of individuals and teams.
- Improve compliance.
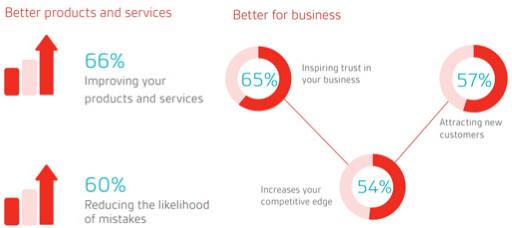
Image Reference: bsigroup.com
What does a QMS need?
A quality management system requires many important elements. Here are few of them:
- Documented quality policy and quality objectives.
- A quality manual which will document scope, exclusions with justification. QMS includes documented procedures, guidelines, checklists. This will allow observed quality and continuous improvement.
- Document procedures mandated by the compliance standard.
- Documents required by the company for effective planning, operation, monitor, and control.
The Importance of Hierarchical Organization
An organization is spirited when working with controlled documents. A suggested hierarchy for QMS documentation management is:
- Quality Manual
- Policies
- Procedures
- Work Instructions
- Lists
- Checklists
- Forms
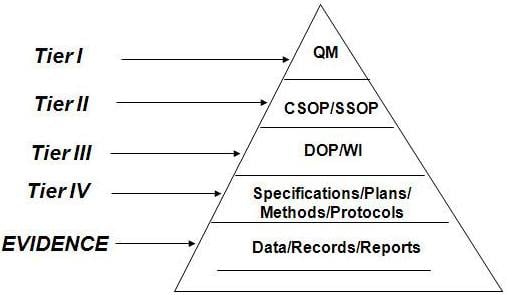
Image Reference: slideshare.net
Steps for the Creation of an Effective QMS
The steps required for the conceptualization and implementation of a QMS include the following:
1. Define and Map Your Processes
Process maps creation will force the organization to visualize and define their processes. In the process, they will define the interaction sequence of those processes. Process maps are vital for appreciating the responsible person. Define your main business process and converse the flow.
Image Reference: flowhelp.com
2. Define Your Quality Policy
Your Quality Policy communicates the duty of the organization as it is about the quality. The mission may be what customers need, a quality mission. When constructing quality management system, consider the commitment towards customer focus. It may be Quality, Customer Satisfaction, and Continuous Improvement.
3. Define Your Quality Objectives
All Quality management systems must have objectives. Each employee must appreciate their influence on quality. Quality objectives are derivative of your quality policy. It is measurable and set up throughout the organization.
The objective may be in the form of critical success factors. This helps an organization in emphasizing the journey towards accomplishing its mission. These performance-based measures deliver a gauge to determine compliance with its objectives.
Some Critical Success Factors are:
- Financial Performance
- Product Quality
- Process Improvement
- Customer Satisfaction
- Market Share
- Employee Satisfaction
4. Develop Metrics to Track and Monitor CSF Data
Once critical success factors are known, measurements and metrics keep track of advancement. This can be done through a data reporting procedure used to collect specific data. Share the processed information with leaders. A process goal is to enhance customer satisfaction index score. There needs to be a goal and a measure to establish achievement of that goal.
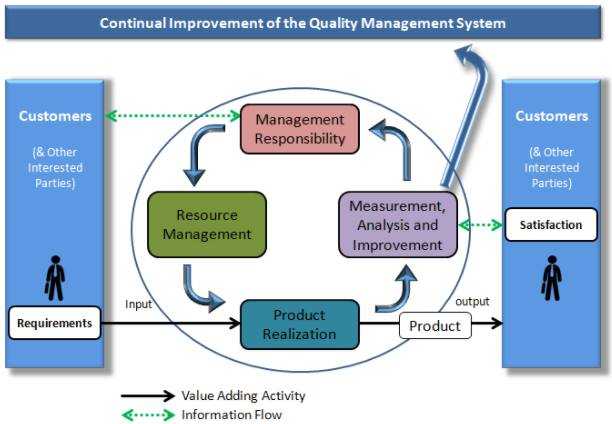
Image Reference: cqeacademy.com
5. Define Defects for Every Process
Defects are non-conformances that happen as a product flaw or a process deficiency. Whenever a defect occurs it needs to be measured and corrected. Identify the required corrective action. When defining your defects:
- Determine operation volume
- Determine defects in product and process
- Define a process to record defects
- Define a process to report defects in specified formats
6. Develop Documents and Records
QMS needs to have some documented information and formats. Start with the minimum required document set and add when needed.
- Create mandatory document information as per business model
- Create essential Quality policies, procedures, and forms
- Create documented information and formats (records) for each defined process
7. Define Quality Process
Your quality procedure includes internal audit, Management review, Corrective and preventive action process and communication processes.
8. Determine Training Needs
Everyone needs to exhibit competency in the job. Training is only the start and can happen on the job, it can be a classroom or e-learning. Some important training areas are- Internal auditor competence, Corrective Action training. Failure Modes Effects Analysis (FMEA) training.
9. Use Quality Management System
Using the QMS means producing the best quality product. In the process
- Collect non-conformance and record them.
- Review this data for corrective and preventive action.
- Review FMEAs for risk and actions, as and when required.
- Perform internal audits and conduct management reviews.
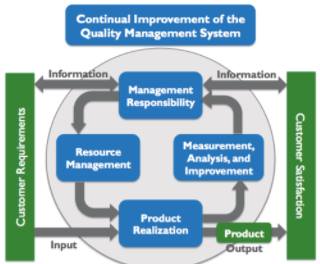
Image Reference: vgblog.wordpress.com
10. Measure, Monitor and implement activities to Improve the Performance
Using quality management system means collecting data. Analyze this data to check if the collected data is good to use and if the intended results can be derived. You need to:
- Track Quality Objectives and its performance
- Define few new performance yardsticks
- Determine improvement chances in the data by recognizing trends, patterns, or correlations.
If you have identified trends through data, then it is time to act. The goal is to bring improvement and this occurs by:
- Arranging your improvement opportunities
- Choosing prospects that make a difference
- Supporting ‘commitment to quality’ to attain better results
Tools to be used in QMS
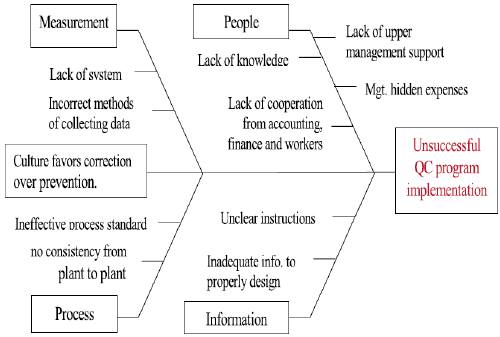
Image Reference: https://www.researchgate.net
Process Maps or Flow Charts
It is important to create a flowchart (process map), it is a popular statistical tool. It helps in streamlining the overall manufacturing process. Easy to understand and very useful, it helps in the visualization of the entire process. It helps in identifying all possible problems that may occur. A properly constructed flow chart showcases quality checkpoints, documentation expectations. It also shows other parts of the formal control plan.
The control plan is approved or signed off by quality assurance leaders. All concerned departments approve the final process steps and flowchart. The critical steps of FMEA come thereafter.
A Closer Look at FMEA
FMEA helps in the identification of potential failures. It also identifies the team’s ability to apprehend and detect failures. It is important for the FMEA team to estimate these factors and rate them on a scale of one to 10. Then, they identify high-risk priority numbers (RPN). RPN forms a platform for the development of all written quality procedures as well as controls. Thereafter, measurements are taken on the basis of RPN results. It happens to the manufacturing process.
Citing an example, NASA had used FMEA for identifying the potential failures in the process of getting US astronauts to the moon and returning them safely back to Earth.
Measuring Systems and Tools
For assuring greater accuracy levels, calibration is required. It is to ensure that measuring systems refrain from inducing errors in the involved processes. A proper calibration of manual as well as electronic measuring devices is a must. Especially for pre/ post- calibration readings that are recorded for more critical features. The measurements conducted at this stage are important parts of Gauge Repeatability and Reproducibility. These are generally expressed in the form of a percentile of measurement error.
While certain quality management systems use Six Sigma for removing the causes of defects/ errors. Six Sigma is used for minimizing variations in manufacturing processes. Others include trace audits and varied statistical tools for reducing costs and increasing profits.
Role of Current Good Manufacturing Practices [cGMP]
Most businesses need assurance that their products and processes follow the specific requirements. Also for requirements of identity, strength, and purity. The creation, implementation, and maintenance of cGMP compliance is a tough task. Involvement of a devoted management team, routine audits, and committed employees is a must. cGMP also requires a well-written and effective quality management system. Companies that follow these regulations presume their suppliers and vendors to do the same.
Conclusion

Image Reference: http://vertoconsulting.eu
Make sure every employee understands the vision. Look for methods to guarantee that all internal processes are consistent. Employees must have the training to comprehend the standardization.
Successful quality enterprises need continuing Senior Leadership. It needs support through a well-defined structure, processes, and transitions. Stakeholder engagement is essential to the effective application of a quality management system.
Click Here for Six Sigma Combo Course
